T/NK Cell
Tumor Cell
CD226
CD155
CD112
CD155
CD112
PD-L1
PD-1
TIGIT
CD226
CD96
PVRIG
PD-1
CD226
CD96
T/NK
Cell
Tumor
Cell
THE CD226 AXIS
Tumors are recognized by the immune system and their progression can be controlled or stopped through a natural process known as immunosurveillance. 1,2
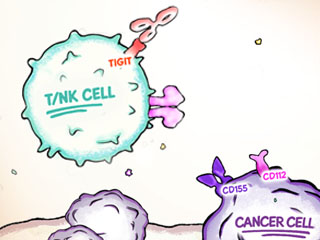
The CD226 Axis includes the T- and natural killer (NK)-cell inhibitory receptors TIGIT, CD96, and PVRIG (CD112R); the T/NK-cell activating receptor CD226 (DNAM-1) ; and cognate ligands CD155 (PVR) and CD112. This multifaceted group of immune receptors plays a critical role in the regulation of antitumor responses. 3,4
THE ROLE OF CD226
CD226 is expressed on the surface of T and NK cells and is involved in the recognition and lysis of a wide range of solid tumors. 2,5,6
CD226 binds to CD155 and CD112, expressed in high levels on tumor cells and on myeloid cells/antigen presenting cells, to stimulate an immune response. 3,5-7
REGULATION OF CD226
The immune checkpoints TIGIT, CD96, and PVRIG (CD112R) may prevent CD226 from interacting with CD155 and CD112, directly impairing NK- and T-cell function; this results in a reduced antitumor immune response. 5
TIGIT
TIGIT is a multifunctional immunoregulatory receptor. 4,5
In addition to intrinsic inhibitory signaling, TIGIT sequesters CD155 and, to a lesser extent, CD112 from CD226, effectively attenuating T- and NK-cell antitumor immune responses. 4,8
TIGIT is highly expressed by immunosuppressive T cells (Tregs) and promotes tumor immune evasion by enhancing Treg function. 4,5,9
TIGIT
Treg Cell
PVRIG
PVRIG is an immune receptor that suppresses T- and NK-cell function to promote tumor immune evasion. 3,4
PVRIG sequesters CD112 from the costimulatory receptor CD226, effectively attenuating antitumor immune responses. 4,5
In addition to ligand competition, PVRIG has also been shown to directly impair immune activation following CD112 engagement. 12
IMMUNE EVASION
Tumors often co-opt normal pathways to their advantage and escape immune surveillance by inducing regulatory pathways, including inhibitory immune receptors. 1,13
The inhibitory effects of TIGIT, CD96, and PVRIG checkpoints, as well as the expression of CD112 and CD155 across tumor types, suggest a broad role in promoting tumor progression and metastasis. 3,5,8,11,14
IMMUNE ENHANCERS
Blocking the TIGIT, CD96, and PVRIG receptors in combination with other checkpoint therapies may enable T and NK cells to better target tumor cells. 3
Preclinical and clinical evidence support targeting TIGIT, CD96, and PVRIG in cancer, especially in combination with other therapies such as those involving PD-1/PD-L1 checkpoint blockade. 3,4,9,15,16
TARGETING THE CD226 AXIS
Current oncology immunotherapies, such as those involving the inhibition of the PD-1/PD-L1 and CTLA-4 pathways, have shown tremendous potential to change cancer treatment. 17
While PD-1/PD-L1 and CTLA-4 blockade can result in dramatic therapeutic responses, these therapies are often effective only in a subset of patients, and those who benefit can develop therapeutic resistance. 17,18
Enhancing natural antitumor responses by blocking inhibitory signaling in the CD226 Axis has the potential to expand the number of patients who respond to immunotherapy treatment and increase the duration of their response, which could bring the promise of effective immunotherapies to more patients with cancer. 3,4
Medical Resources
The
CD226 Axis
Its Role in Tumor Immunosuppression and Its
Potential as a Target for New Immunotherapies
THE CD226 AXIS
THE ROLE OF CD226
REGULATION OF CD226
IMMUNE EVASION
IMMUNE ENHANCERS
TARGETING THE CD226 AXIS
EDUCATIONAL RESOURCES
Videos
Other Resources
References
Abbreviations
Cookie Policy
Legal Notice
Privacy Notice
Terms of Use
This is a global website and is intended for healthcare professionals. The information on this site is intended for educational purposes only and is not country-specific.